IAVI is a scientific research organization that develops vaccines and antibodies for HIV, tuberculosis (TB), emerging infectious diseases (EIDs), and neglected tropical diseases (NTDs). This nonprofit organization is committed to its mission to translate scientific discoveries into affordable, globally accessible public health solutions. IAVI collaborates with an extensive network of partners in academia, industry, foundations, governments, nonprofit organizations, communities, and funders.
Twenty-five years of global health milestones
1995
Starting in 1994, the Rockefeller Foundation convenes a series of three international meetings with authorities on HIV/AIDS in Bellagio, Italy, Paris, and New York, respectively, to determine what hampered progress toward the development of preventive HIV vaccines.
At the Bellagio meeting, 24 AIDS authorities from around the world call for a new model of vaccine development to bridge the public and private sectors, focus on LMICs, and link advocacy with accelerated product development. Read the Bellagio Report.
1996
 The International AIDS Vaccine Initiative (IAVI) is established to accelerate the development of safe and effective AIDS vaccines for use throughout the world, supported by the Rockefeller Foundation, Alfred P. Sloan Foundation, Fondation Mérieux, Until There’s A Cure, the World Bank, and UNAIDS. Major initial activities include establishment of its scientific advisory committee to guide targeted vaccine development; an international campaign to create a Global HIV Vaccine Purchase Fund; and consultations with the AIDS community in Europe, North America, and various LMICs to review the state of HIV vaccine R&D and to seek advice on moving the field forward.
The International AIDS Vaccine Initiative (IAVI) is established to accelerate the development of safe and effective AIDS vaccines for use throughout the world, supported by the Rockefeller Foundation, Alfred P. Sloan Foundation, Fondation Mérieux, Until There’s A Cure, the World Bank, and UNAIDS. Major initial activities include establishment of its scientific advisory committee to guide targeted vaccine development; an international campaign to create a Global HIV Vaccine Purchase Fund; and consultations with the AIDS community in Europe, North America, and various LMICs to review the state of HIV vaccine R&D and to seek advice on moving the field forward.
IAVI launches the world’s first newsletter on AIDS vaccine research, IAVI Report.
1997
Seth Berkley, M.D., formerly at the Rockefeller Foundation, becomes IAVI’s president and CEO.
The U.K.’s National AIDS Trust becomes IAVI’s first formal NGO partner.
IAVI makes its first three HIV vaccine R&D awards to groups in Melbourne, Australia, and Boston, USA.
1998
UNAIDS names IAVI as a collaborating center.
Through a grant from the Department for International Development (DFID), the U.K. becomes IAVI’s first government partner.
IAVI unveils the Scientific Blueprint for AIDS Vaccine Development, a new global strategy to fast-track HIV vaccine development. The Blueprint proposes Vaccine Development Partnerships (VDPs) linking scientists in LMICs and HICs.
IAVI launches its first two VDPs, the first in global research to target the strains of HIV commonly found in Africa (clades A and C). These include the University of Nairobi/Kenya AIDS Vaccine Initiative (KAVI) with the U.K. Medical Research Council and another between the American biotechnology company AlphaVax and the University of Cape Town. Read more about this here.
1999
The William H. Gates Foundation (later known as the Bill & Melinda Gates Foundation) awards $25 million to IAVI, its largest charitable AIDS gift to date.
The United Kingdom, IAVI’s first major public sector supporter, awards IAVI £14 million. The Netherlands also commits to funding IAVI.
With a first grant of 5 million Dutch Florins (eq. €2.25 M), the Dutch government becomes the second government donor on board.
2000
Researchers from across Africa release “The Nairobi Declaration: An African Appeal for an HIV Vaccine.”
IAVI enters into a third VDP with Targeted Genetics Corporation and the Children’s Research Institute of Columbus, Ohio, to begin developing a recombinant adeno-associated virus vector (AAV)-based HIV vaccine candidate.
IAVI enters into a vaccine research collaboration agreement with the Aaron Diamond AIDS Research Center, an affiliate of Rockefeller University, in December 2000, to advance HIV vaccine candidates.
Canada and Ireland join the governments of the U.K. and the Netherlands in providing multi-million dollar support to IAVI.
In Durban, South Africa, IAVI unveils AIDS Vaccines for the World: Preparing Now to Assure Access, the first plan to outline the steps needed for simultaneous access to a successful AIDS vaccine in LMICs and HICs, in conjunction with its second Scientific Blueprint.
U.S. Congress authorizes $10 million to IAVI for each fiscal year of 2001 and 2002 through the Global AIDS and Tuberculosis Relief Act of 2000, the first U.S. contribution to IAVI. This directive to the United States Agency for International Development (USAID) from the U.S. Congress marks the beginning of 20+ years of partnership in a shared endeavor to ensure the development of safe, effective, accessible, preventive HIV vaccines and biomedical prevention tools for use throughout the world while strengthening clinical research capacity in regions most devastated by the epidemic. USAID, on the forefront of the global AIDS crisis for more than 30 years and a key implementing agency of the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), invests in the science that will deliver results and translate them from research to practice for populations at risk.
2001
At the United Nations General Assembly Special Session on HIV/AIDS, IAVI launches a “Call for Action,” a petition urging world leaders to “to ensure the development of safe, effective and accessible preventive AIDS vaccines for use wherever they are needed.”
To mobilize global support for IAVI’s Scientific Blueprint, the Bill & Melinda Gates Foundation pledges a US$100 million challenge grant — the largest single award to date.
Yahoo! becomes IAVI’s first major corporate sponsor.
IAVI and the Government of the Republic of Uganda establish IAVI’s fourth VDP to test candidates, strengthen clinical trial infrastructure, and build the capacity of local scientists. This later leads to the creation of the UVRI-IAVI program. The partnership links IAVI and the Uganda Virus Research Institute (UVRI) with researchers at the Institute for Human Virology (IHV) at the University of Maryland in the U.S. and at Oxford University in the U.K., with the goal of testing an IHV/Oxford-developed candidate.
Building on the University of Oxford-University of Nairobi-IAVI partnership, Kenya embarks on the country’s first HIV vaccine clinical trial. The Phase I safety trial aims to test a DNA prime/modified vaccinia virus boost combination of HIV vaccine candidates.
Building on a vaccine collaboration agreement established between IAVI and Therion Biologics in 2000, IAVI establishes a vaccine evaluation agreement with the Indian Council of Medical Research (ICMR) to develop Therion’s multigenic viral vector vaccine (MVA, or modified vaccinia virus Ankara) based on the Clade C subtype of HIV, which predominates in LMICs. The candidate is based on an MVA pox virus vector and is referred to as an MVA-HIV vaccine. The goal of the collaboration is to apply Therion’s vaccine technology and IAVI’s development capabilities and in-country expertise in HIV/AIDS research for rapid, cost-effective development and manufacturing of an India-specific AIDS vaccine.
IAVI establishes an HIV vaccine development collaboration with Bioption, a Swedish biotech, based on Bioption’s SFV vaccine technology (Semliki Forest Virus vector), with an Oxford University HIV A gene insert. The focus of this partnership is to develop the candidate and conduct clinical studies in India.
IAVI launches its activities in India, including its Sankalp newsletter.
The Canadian AIDS Society joins forces with IAVI to build political and public commitment to speed the search for an AIDS vaccine for the hardest-hit countries.
IAVI’s partnership with USAID begins. This announcement marks the beginning of 20+ years of partnership in a shared endeavor is to ensure the development of safe, effective, accessible, preventive HIV vaccines and biomedical prevention tools for use throughout the world while strengthening clinical research capacity in regions most devastated by the epidemic.
IAVI opens a state-of-the-art Human Core Immunology Laboratory at Imperial College in London to facilitate coordination, quality control, and high-level training among its global partners.
IAVI partners with Brazil’s NGO community and its National AIDS Program to keep vaccines on their agenda and to further collaboration with other countries.
IAVI’s Access Project collaborates with the World Health Organization to publish A New Access Paradigm — a whitepaper assessing potential worldwide demand for HIV vaccines.
With Norway and Denmark’s contributions, the total number of donor countries supporting IAVI’s activities increases to seven.
A vaccine preparedness program is launched to work with in-country partners to build and sustain an enabling environment in LMICs hosting clinical trials.
2002
Berna Biotech of Switzerland joins the Institute of Human Virology vaccine development partnership to test an orally administered AIDS vaccine.
The University of Oxford-University of Nairobi-IAVI partnership moves the DNA-MVA vaccine into second-stage trials in the U.K.
Swedish biotech firm Bioption AB teams with IAVI to develop an HIV vaccine using the proprietary technology developed by the Karolinska Institute, SFV alphavirus replicons.
IAVI’s Vaccine Preparedness program and partners convene parliamentarians and AIDS program implementers from nine countries in New Delhi, leading to a new level of political commitment to fight AIDS in India.
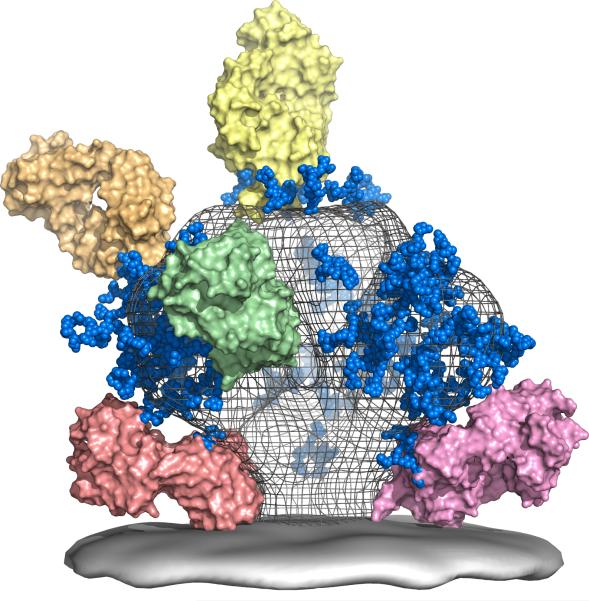
The Scripps Research Institute, Weill Medical College of Cornell University, University of Pennsylvania, Dana-Farber Cancer Institute, and IAVI form the Neutralizing Antibody Consortium (NAC) to address a core HIV vaccine challenge: eliciting antibodies that neutralize a broad range of HIV strains, known as broadly neutralizing antibodies (bnAbs). The Dale and Betty Bumpers Vaccine Research Center at the National Institute of Allergy and Infectious Diseases, a part of the US National Institutes of Health, also agrees to support the NAC’s efforts. It’s the first major scientific collaboration to try to unravel the mysteries of neutralizing antibodies against HIV.
IAVI opens regional offices in Amsterdam and New Delhi.
At the Barcelona AIDS Conference, IAVI outlines its new R&D agenda to expand the pipeline of candidate AIDS vaccines in final-stage human efficacy trials.
IAVI constructs new laboratories — centers of excellence — to support future vaccine trials in Pune, India, and at UVRI in Entebbe. A second Indian laboratory is later opened in Chennai.
2003
IAVI establishes its Policy Advisory Committee and receives its first European Commission funding for vaccine preparedness programming in East Africa.
In Africa, the DNA-MVA vaccine begins Phase I safety testing in Uganda through IAVI partners at UVRI; a regional office is opened in Nairobi to support IAVI’s East African activities; the UVRI-IAVI HIV Vaccine Program is inaugurated; and South Africa approves its first HIV vaccine trials, including one sponsored by IAVI.
IAVI launches VAX, a monthly bulletin of information about AIDS vaccine research, clinical trials and associated subjects.
IAVI participates in the Good Clinical Laboratory Practice (GCLP) project to finalize the world’s first guidelines “that set a standard for compliance by laboratories involved in the analysis of samples from clinical trials. These guidelines provide guidance to ensure that trial laboratory data are reliable, repeatable, auditable, and easily reconstructed in a research setting.”
Targeted Genetics, Columbus Children’s Research Institute, and IAVI begin IAVI A001 — human trials of a single-shot vaccine (tgAAC09) — in Belgium. The vaccine construct is an HIV-1 clade C Adeno-associated virus (AAV) capsid.
2004
IAVI terminates development of its DNA-MVA candidate after Phase II clinical data do not meet predetermined criteria to advance Phase Ill (efficacy) trials. Separately, IAVI terminates product development of the SFV replicon candidate based on preclinical data.
IAVI expands it public policy program to mobilize public support for AIDS vaccine incentives for industry and assistance for LMICs to test, plan vaccine distribution at affordable prices.
The IAVI Core Laboratory in London is the first laboratory in the world to receive full GCLP accreditation, the global gold standard.
In its Scientific Blueprint 2004, IAVI calls for a doubling of current spending on AIDS vaccine development (then at $650 million) to help broaden the diversity of approaches in testing.
With IAVI support, four African research teams, two in Kenya and two in Uganda, begin epidemiological studies in Kangemi, Nairobi, to screen a high-risk cohort of volunteers to examine HIV prevalence. This will later expand and evolve to support prospective cohorts to better understand HIV incidence and volunteer retention.
The Dutch biotech firm Crucell N.V., grants IAVI exclusive license to use its AdVac adenovirus vectors to develop an AIDS vaccine. Crucell later becomes part of Janssen Pharmaceutical Companies and then Johnson & Johnson.
IAVI launches the Live Attenuated Consortium (LAC) to accelerate vaccine candidates that will confer same level of protection as mutated live attenuated SIV in non-human primates.
2005
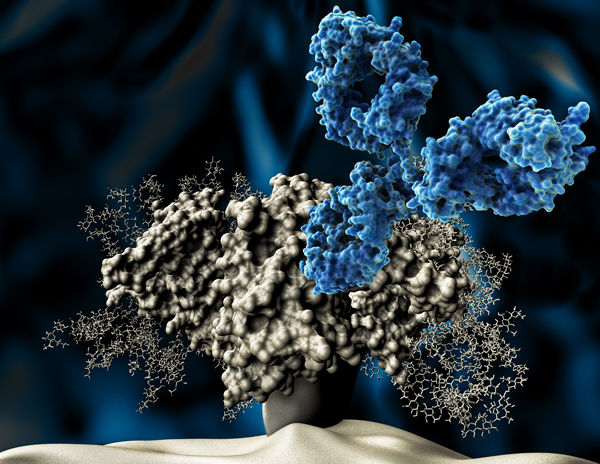
The Neutralizing Antibody Consortium (NAC) solves the crystal structure of monoclonal antibody 4E10, the most broadly neutralizing antibody discovered to date.
(Image from Science 13 Jul 2012: Vol. 337, Issue 6091, pp. 183-186. Reprinted with permission from AAAS)
The National AIDS Research Institute (NARI), in partnership with the Indian Council of Medical Research and IAVI, begins the country’s first Phase I clinical trial of a preventive AIDS vaccine (tgAAC09) in Pune, India. This is followed by two more preventive vaccine trials at NARI and National Institute for Research in Tuberculosis (NIRT), in 2006 and 2009.
IAVI opens an R&D lab in New York — the first applied research, industrial-style AIDS vaccine development laboratory by a not-for-profit organization. In 2008, this lab is publicly launched as the IAVI Design and Development Laboratory in Brooklyn.
In its first collaboration with a major vaccine company, IAVI partners with GlaxoSmithKline (GSK) Biologicals to develop AIDS vaccines based on chimpanzee adenoviruses and IAVI’s human adenovirus vaccine candidates.
Through an IAVI/Targeted Genetics partnership, three sites in South Africa launch the first Phase Il vaccine trial (IAVI A002) in that country. The vaccine construct is an HIV-1 clade C AAV capsid.
IAVI’s first AIDS vaccine trial (IAVI V001) is launched through a partnership between Emory Projet San Francisco, IAVI, and the NIH. The trial aims to test two VRC-developed candidates: a DNA vaccine followed by an adenoviral vector vaccine.
IAVI’s first modeling estimates of the potential impact of an AIDS vaccine globally and by region are published for World AIDS Day and quickly become the standard reference for estimates on the benefits of a vaccine.
2006
IAVI signs its first five-year, $155 million cooperative agreement with USAID to support IAVI’s research, clinical trials, and policy analysis.
The U.S. House of Representatives passes, by unanimous consent and with strong bipartisan support, a resolution congratulating IAVI on its significant achievements in the search for an HIV/AIDS vaccine.
UVRI, IAVI, and Targeted Genetics initiate a Phase Il trial (IAVI A002) in Entebbe, Uganda. The trial also marks, in a partnerships with the Zambia Emory HIV Research Project (ZEHRP), the first preventive HIV vaccine trial in Zambia.
KAVI, IAVI, and the NIH’s Vaccine Research Center launch trials in Nairobi, Kenya, using a VRC-designed candidate at a site developed by KAVI and IAVI (IAVI V001).
India’s second AIDS vaccine trial (IAVI A001) is launched in Chennai, through the ICMR, the National AIDS Control Organization, and IAVI.
IAVI grants Beth Israel Deaconess Medical Center a license to use IAVI’s adenovirus-based vaccine production technology in its HIV vaccine research program.
IAVI’s paper Putting It Together: AIDS and the Millennium Development Goals (MDGs) is published for the UN Special Session on the MDGs, and rapidly becomes its most widely sought policy paper, distributed by UNAIDS and major AIDS NGOs.
With IAVI support, the Kenya Medical Research Institute opens two new buildings in Kilifi to facilitate HIV vaccine research, help prepare for future trials, and ensure access to enhanced HIV care (including counseling and education).
The UVRI-IAVI lab in Entebbe, Uganda, is fully GCLP-accredited — the first lab in sub-Saharan Africa (outside of South Africa) to receive this distinction. The KAVI-Kangemi field lab in Nairobi, Kenya, follows.
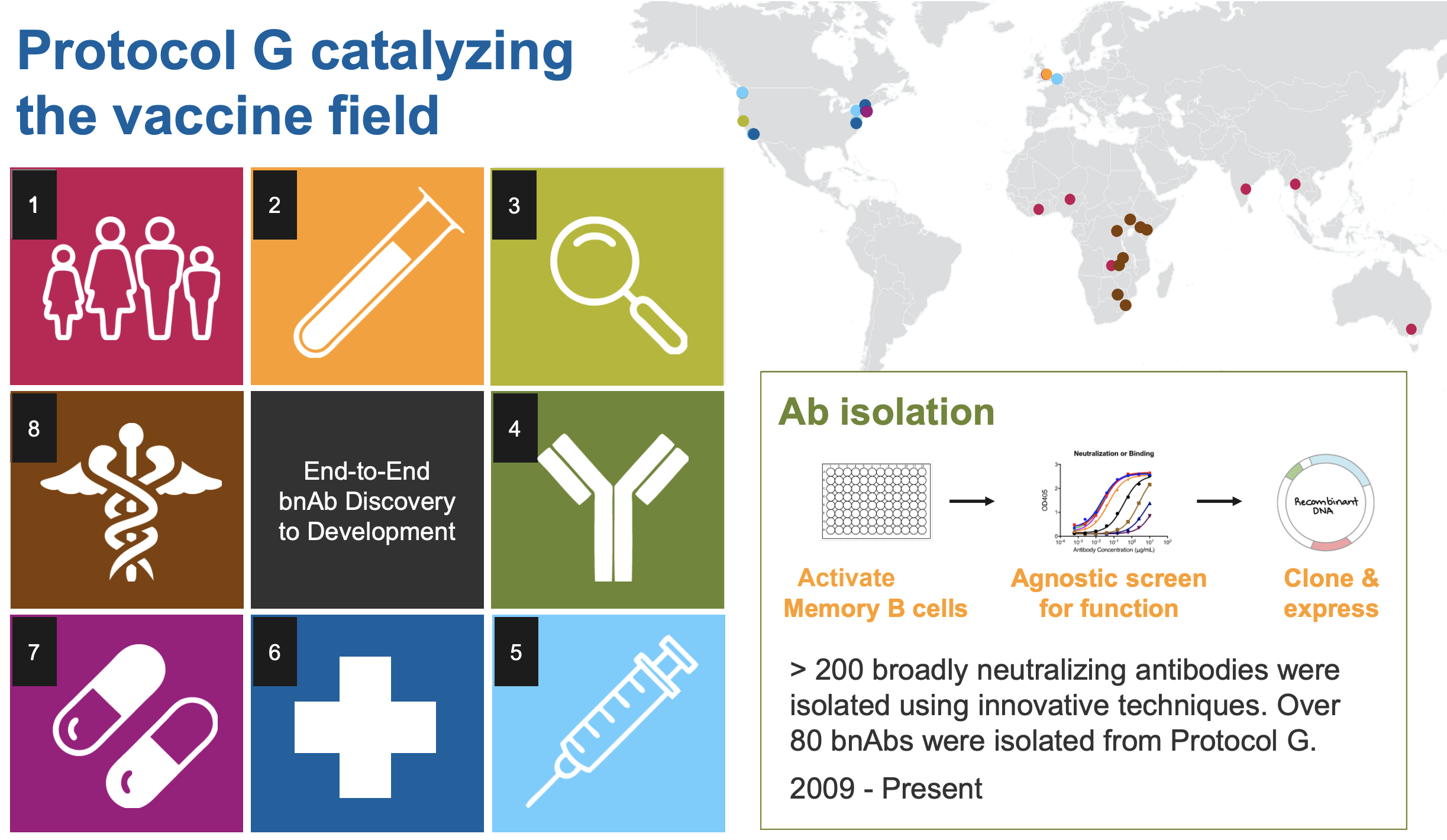
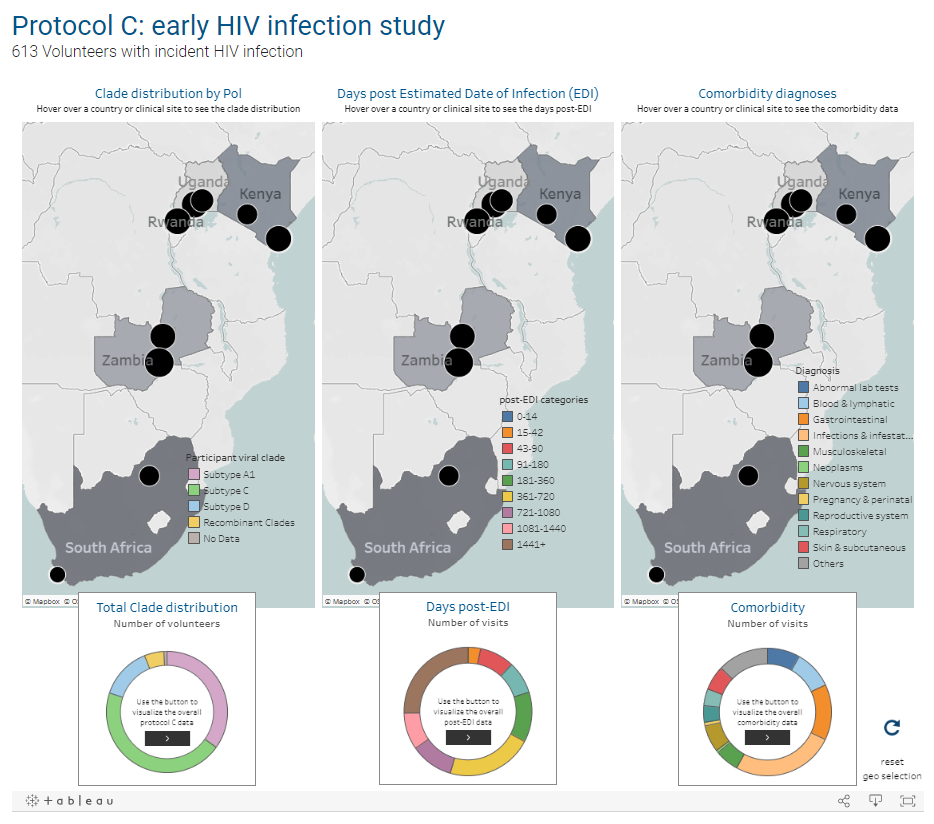
USAID-supported Protocols C, D, and G begin. C is the largest prospective study of early HIV infection in African adults, to better understand the complex interaction between HIV and the immune system. D is a cross-sectional study to characterize laboratory reference intervals in healthy African adults toward improving participation of African volunteers in clinical trials for new drugs and vaccines against diseases such as AIDS, TB, and malaria. G is a cohort study to identify new bnAbs in adults in Africa, Asia, Australia, the U.K., and the U.S.
As an enabling resource for the NAC, Scripps Research Institute and IAVI unveil a robot that allows rapid determination of the molecular crystal structure of the HIV envelope, accelerating vaccine design efforts.
IAVI receives $23.7 million from the Bill & Melinda Gates Foundation, one of 16 grants awarded to establish collaborative research consortia focused on accelerating the pace of HIV vaccine development. IAVI also receives a total of $9.46 million from grants awarded to Duke University and the NIH for quality control laboratory activities in support of other Gates Foundation-funded vaccines in clinical trials.
2007
There are now more than 30 HIV vaccine clinical trials ongoing in 24 countries, across every continent, according to IAVI’s VAX Report.
2008
Scripps Research and IAVI launch the HIV Neutralizing Antibody Center (NAC) located at Scripps in La Jolla, California, and linked to a network of research institutions in Africa, Asia, Europe, and the U.S.
IAVI Vaccine Design & Development Laboratory (DDL) opens in New York City to accelerate development of promising HIV vaccines from concept to clinic and to further research on novel replicating viral vector technology for delivering candidate HIV vaccines.
The IAVI-Africa clinical research center (CRC) partner network expands to include the Aurum Institute in Rustenburg, South Africa.
The Indian Council of Medical Research, the National AIDS Control Organization, and IAVI announce the successful completion of the Phase I MVA-based AIDS vaccine candidate known as TBC-M4.
IAVI and the St. Stephen’s AIDS Trust at the Chelsea and Westminster Hospital start a Phase I clinical trial to test a prime-boost combination of two HIV vaccine candidates in the U.K.
2009
TIME magazine names IAVI President & CEO Seth Berkley one of the “100 Most Influential People in the World.”
Scripps, IAVI, Theraclone Sciences, and Monogram Biosciences discover first new bnAbs — PG9 and PG16 — to have been identified in over a decade. Both were isolated from the blood of an African donor living with chronic HIV infection as part of the USAID-supported Protocol G. The results provide a framework for the design of new vaccine candidates for the elicitation of bnAb responses.
The IAVI Neutralizing Antibody Center laboratories are formally established at Scripps Research campus.
2010
The Government of Japan pledges US$10 million to support IAVI’s AIDS vaccine research and development over the next five years. The grant focused on the development of a vaccine platform technology for an HIV vaccine candidate, in partnership with a Japanese biotech company, DNAVEC.
IAVI NAC receives NIH funding to investigate the biological mechanisms underlying the generation of broadly neutralizing antibodies by HIV positive individuals.
The IAVI B003 clinical trial begins to test the safety and immunogenicity of two preventive AIDS vaccine candidates in a prime-boost combination.
The IAVI-Africa CRC partner network expands to include the University of KwaZulu-Natal HIV Pathogenesis Programme in Durban, South Africa.
2011
Margaret McGlynn named IAVI President and CEO, replacing IAVI founder Seth Berkley.
IAVI and its partners receive a second five-year (2011-2016), $160 million cooperative agreement with USAID provided through PEPFAR to support IAVI’s research, clinical trials, and policy analysis.
In partnership with GSK and KAVI, IAVI begins the Phase I B002 HIV vaccine multi-center clinical trial to test a combination of two experimental HIV vaccine components in Kenya, Uganda, and Zambia. The vaccine components are a protein (F4co) and an adenovirus 35 vector expressing HIV proteins.
amfAR, IAVI, the John Hopkins University Center for Public Health and Human Rights, and the United Nations Development Program release Respect, Protect, Fulfill at the 16th ICASA Conference in Addis Ababa, Ethiopia. The document is updated in 2015 to provide guidelines for organizations conducting research with men who have sex with men, LGBTQ populations in criminalized and rights-constrained settings.
The Phase I IAVI B003 clinical trial of Ad 26 and Ad35 vectored HIV vaccines begins in Rwanda, Kenya, and South Africa.
2012
IAVI-Translational Health Science and Technology Institute (THSTI) HIV Vaccine Translational Research Laboratory opens in India to study genetic and virological properties of circulating HIV strains in India.
IAVI and Aeras partner to share knowledge and resources across the clinical trials networks of both organizations.
The Phase I IAVI B004 clinical trial of a DNA-based HIV vaccine begins in Kenya, Uganda, and Rwanda.
Females Rising through Education, Support and Health (FRESH) study to identify/enroll volunteers with acute HIV infection in in Durban, South Africa begins. The FRESH study follows young women at high risk of HIV infection, and combines research and social support. Women receive life- and job-skills training, prevention counseling, and testing to diagnose new HIV infections early. IAVI’s collaboration with the University of KwaZulu-Natal’s HIV Pathogenesis Programme (HPP) is focused on providing support to the HPP FRESH project, recruiting more than 2,000 young women (18-23 years old).
2013
The Bill & Melinda Gates Foundation awards a grant to IAVI to create the Vaccine Product Development Center (VxPDC) to assist investigators affiliated with the Foundation’s Collaboration for AIDS Vaccine Discovery with the complex process of transitioning vaccine candidates from the laboratory to the clinic. Subsequently, IAVI expanded the Product Development Center (PDC) in recognition of efforts beyond vaccines and extending this translational expertise to other partners. The PDC fills a critical gap in the development process for new vaccines and other biomedical innovations.
NAC scientists apply computational and genetic engineering techniques to generate a virus-like particle (eOD-GT6) that activates bnAb precursors in the lab.
IAVI launches S001, the first HIV vaccine clinical trial to use the Sendai virus as a replicating viral vector, in Rwanda.
Two landmark studies supported by IAVI and the NIH describe the structure of the outer part of the HIV trimer, the tripartite HIV Envelope protein — achieving a decade-long goal in HIV vaccine research.
IAVI supports the establishment of two, multi-team consortia to address the specific needs of key and priority populations in east Africa. These include the Men’s Health Research Consortium in Kenya, which links together three teams studying health issues relevant to men who report sex with men and transgender women, and the Lake Victoria Consortium for Health Research, whose mission is to improve the health of the fishing communities on Lake Victoria shoreline and islands in Kenya, Tanzania, and Uganda.
With funding from the Robert Wood Johnson Foundation, IAVI launches the Human Vaccines Project to accelerate the development of new vaccines against AIDS, tuberculosis, malaria, and other major diseases.
2014
IAVI launches IAVI A003, a Phase I clinical study of a prototype candidate for preventing HIV using vectored gene delivery of a bnAb.
The Phase I N004 (HIVCORE 004) clinical trial of DNA and MVA based conserved vaccine begins in Nairobi.
NAC scientists identify a fifth vulnerable binding site on the surface of HIV and two bnAbs capable of blocking infection by more than two-thirds of HIV strains found in patients worldwide.
With support from USAID through PEPFAR, IAVI launches the Vaccine Immunology Science and Technology for Africa (VISTA) and the International Training Program to strengthen research capacity for HIV vaccine research in sub-Saharan Africa.
Establishment of the national Sexual Orientation Gender Identity and Expression (SOGIE) Research Advisory Committee (G10) in Kenya and inclusion of the G10 as an advisory member to the National AIDS and STI Control Programme (NASCOP) technical working group to advise and provide leadership on issues related to MSM and LGBTQ research nationally.
IAVI influences and supports the development of the National Guidelines for HIV/STI Programming with Key Populations in Kenya.
2015
IAVI receives a grant of US$350,000 from GSK to support implementation of the Human Vaccines Project, a new public-private partnership seeking to transform global disease prevention by collaboratively addressing some of the key scientific challenges in vaccine development across diseases.
IAVI names Mark Feinberg, M.D., Ph.D., as president and CEO.
A study published in RAND Europe reports that IAVI has made significant contributions to strengthening health research systems in East Africa.
IAVI begins to provide support to the European AIDS Vaccine Initiative (EAVI2020), financed by the European commission.
IAVI supports and collaborates with Kenya’s National AIDS and STIs Control Programme (NASCOP) and other partners to develop and launch Guidelines for Conducting Adolescent HIV Sexual and Reproductive Health Research in Kenya.
2016
IAVI CEO Mark Feinberg, M.D., Ph.D., begins diversification of IAVI beyond HIV for broader global health impact.
IAVI and its partners receive a five-year cooperative agreement award with a US$160 million ceiling from USAID provided through PEPFAR. Building on long-standing partnerships with USAID and African research centers, this new program to Accelerate the Development of Vaccines and New Technologies to Combat the AIDS Epidemic (ADVANCE) aims to advance the design and development of HIV vaccines and biomedical prevention tools while ensuring they are effective and accessible for all in need.
After an initial Declaration draft failed to reference preventive vaccines, IAVI leads on a joint letter to the UN leadership (co-signed by AVAC, HVTN, MHRP, NIAID, GHVE, Population Council) during the UNHLM on Ending AIDS.
The collaboration for AIDS Vaccine Discovery, funded by the Bill & Melinda Gates Foundation, awards IAVI a grant to expedite the development of around a dozen promising AIDS vaccine candidates or biologics toward clinical testing. The National Institute of Allergy and Infectious Diseases (NIAID), part of the U.S. National Institutes of Health (NIH), awards IAVI a contract for product development services to advance the development of AIDS vaccine candidates of NIAID-supported scientists.
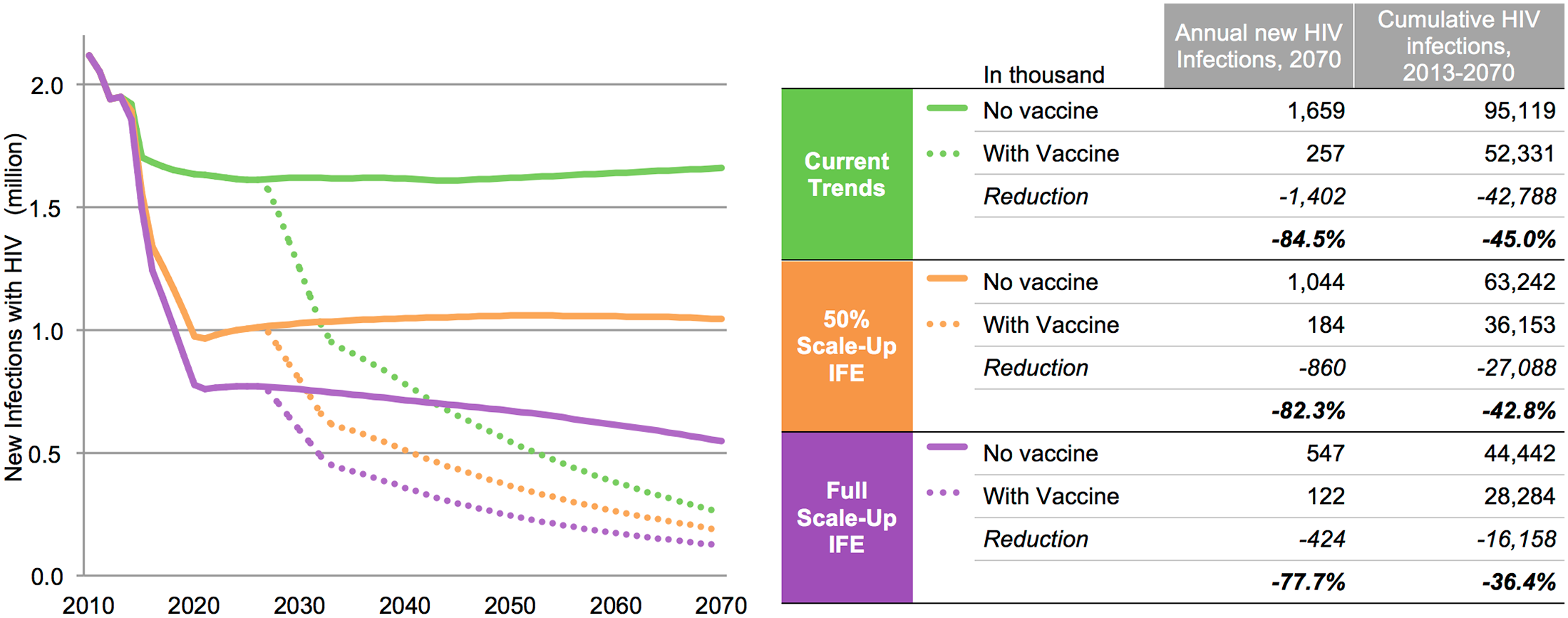
A modeling study conducted by IAVI, AVAC, and Avenir Health and published in PLOS One shows that a vaccine is essential to ending HIV/AIDS.
Funded by a European Union grant under the Horizon 2020 health program, IAVI joins the European HIV Vaccine Alliance, a consortium to accelerate new concepts in HIV vaccine research.
The Netherlands Ministry of Foreign Affairs awards €16 million to IAVI over five years.
The Phase I IAVI B003 HIV vaccine clinical trial concludes.
Establishment and launch of the G10 Research Engagement Roadmap (2016/17–2018/19) and the Integrated Community Online Platform (ICOP). The roadmap and ICOP provided a framework by which researchers, policymakers, and funders could engage the G10 on various capacity strengthening initiatives, while the ICOP is an online platform that enables the members of the LGBTQ communities in Kenya to connect locally, regionally, and globally and increase the visibility of their work.
2017
A series of discoveries by NAC scientists published in leading journals, including PLOS Pathogens and Science, further support structure-based HIV vaccine design strategies and exploration of bnAbs as long-acting HIV prevention.
IAVI, Oxford, KAVI, UVRI-IAVI, KEMRI-Wellcome, MRC/UVRI/LSHTM, and the Rwanda Zambia Health Research Group establish the Globally Relevant AIDS Vaccine Europe-Africa Trials Partnership (GREAT) to develop a broadly effective AIDS vaccine candidate.
In collaboration with the Beth Israel Deaconess Medical Center, IAVI begins a Phase I clinical trial of an HIV bnAb infusion PGT-121 to prevent HIV infection (IAVI T001).
IAVI partners with the National AIDS Control Council (NACC) in Kenya to develop and launch the National Violence Prevention and Response Protocol: A means to Enhancing HIV Prevention among Key Populations toward reducing and eliminating violence aimed at key populations. IAVI also supports the launch of the NACC’s Maisha Maarifa online research hub.
IAVI partners with ICMR and the Department of Biotechnology to set up the National HIV Cohort Program, a first-of-its-kind effort in India to bring together institutions of national repute for the systematic, long-term, and structured study of HIV in Indian populations of interest.
2018
IAVI begins the first clinical trial (IAVI G001) to test a novel vaccine candidate (eOD-GT8 60mer) designed to stimulate the immune system to initiate a key first step in the generation of bnAbs. IAVI and Scripps scientists at the NAC designed the candidate — a decade of discovery coming to fruition.
IAVI expands into vaccines against emerging infectious diseases, including Lassa fever. The newly created Coalition for Epidemic Preparedness Innovations (CEPI) and IAVI begin a partnership to develop rVSVΔG-LASV-GPC, a replicating viral vector-based vaccine candidate against Lassa fever virus, together with a consortium of partners in west Africa. IAVI licensed the vaccine technology underlying rVSV∆G-LASV-GPC from the Public Health Agency of Canada (PHAC). rVSV∆G-LASV-GPC is based on an rVSV vector and was developed by scientists at PHAC’s National Microbiology Laboratory.
IAVI and the Serum Institute of India partner to develop and manufacture globally affordable and accessible antibody products for HIV.
Aeras TB transfers TB vaccine clinical programs and assets to IAVI. The transfer coincides with a New England Journal of Medicine publication showing that GSK’s investigational TB vaccine candidate, M72/AS01E, developed with Aeras, has an overall vaccine efficacy of 54 percent. It’s the first new TB vaccine to show efficacy in 100 years.
IAVI establishes a collaborative relationship with the Gates Medical Research Institute in certain areas of the former Aeras TB portfolio programs.
IAVI supports development of the National Implementation Guidelines for HIV and STI Programming among Young Key Populations in Kenya and the Roadmap to Accelerate HIV Combination Prevention in Fishing Communities in Uganda. In addition, IAVI partners with the MSM Health Research Consortium (MHRC) to launch a website so that MHRC-funded work is widely available.
IAVI, Delhi Dance Theater, and PULSE ensemble collaborate to produce “I AM +,” a first-of-its kind, immersive dance theater performance to communicate the critical need for collaborative and integrated efforts to better understand the challenges of HIV infection.
2019
USAID announces it will continue to support the ADVANCE program through 2026 with a $160 million extension of the 2016 cooperative agreement.
IAVI announces the start of a Phase I clinical trial (IAVI W001) of a native-like HIV envelope vaccine candidate (BG505 SOSIP.664 gp140) in Nairobi, Seattle, and Boston. The candidate was developed based on a virus isolated from a Kenyan infant two decades earlier.
IAVI’s antibody program expands to include development of next-generation snakebite treatment as part of The Scientific Research Partnership for Neglected Tropical Snakebite (SRPNTS), a U.K.-funded consortium co-led by the Liverpool School of Tropical Medicine and with partners on four continents.
Supported by IAVI and the MHRC, members of the G10 initiate the first-ever community-led Mtazamo Mpya study to compare actual and perceived sexual risk among LGBT persons in Kenya.
The results of the GSK/IAVI Phase IIb trial of M72/AS01E1 TB candidate vaccine were published and showed a significantly reduced incidence of pulmonary tuberculosis disease (TB) in HIV-negative adults with latent TB infection who received the vaccine. These results demonstrate an overall vaccine efficacy of 50% during the three years after vaccination. In partnership with European funders, IAVI also announces collaborations in two other TB vaccine clinical trials initiated this year (MTBVAC and H56:IC31) — both in sub-Saharan Africa.
IAVI Report publishes a special issue on the increasingly prominent role African scientists are playing in the quest to develop an HIV vaccine. Much of this progress is the result of a sustained effort by IAVI, through its decades-long partnership with USAID, to nurture scientific talent on the African continent.
The Research Council of Norway through the GLOBVAC program funds IAVI, THSTI, and the University of Oslo to develop HIV bnAbs as a prevention product for affordable global access.
The Defense Threat Reduction Agency (DTRA) of the U.S. Department of Defense funds IAVI to develop an rVSV-based vaccine for Marburg virus, a potential bioterrorism threat.
2020
Thousands of Protocol C samples are made available to researchers through the IAVI DataSpace. This study and two other related studies are later profiled in the International Journal of Epidemiology for their contributions to addressing gaps in HIV epidemiology and vaccine research.
IAVI and Merck announce a collaboration to develop a vaccine against SARS-CoV-2 using recombinant vesicular stomatitis virus (rVSV) technology that is the basis for Merck’s Ebola Zaire virus vaccine. The Government of Japan and the U.S. Department of Defense pledge funds to the effort. Scientists at the DDL in Brooklyn lead preclinical development of the vaccine candidate.
IAVI, Scripps Research, and the NIH agree to pool their HIV bnAb assets and expertise to develop a combination bnAb product specifically designed to be available promptly, affordably, and sustainably globally.
IAVI NAC and Scripps Research scientists are part of a team that identifies antibodies from the blood of recovered COVID-19 patients that are capable of potently neutralizing SARS-CoV-2, the virus that causes COVID-19. Animals that received these neutralizing antibodies were protected against disease after challenge with SARS-CoV-2. The results are published in Science.
IAVI and Wellcome issue “Expanding Access to Monoclonal Antibody-Based Products: A Global Call to Action,” urging global health leaders to make monoclonal antibodies globally accessible.
IAVI announces launch of a European & Developing Countries Clinical Trials Partnership (EDCTP)-funded Socio-behavioral Project on Accessible HIV Prevention Tools for African Women (UPTAKE). The project focuses on adolescent girls and young women in Kenya and Uganda and leverages the expertise of IAVI’s longstanding CRC partners in sub-Saharan Africa.
The Female Sex Workers Research Advisory Committees are formed in three regions in Kenya (Kisumu, Nairobi, and the Coast). These committees are intended to increase the involvement and influence of female sex workers in research and translation of research into policies and practice in Kenya.
With support from CEPI, Wellcome, and many regional and international partners, IAVI participates in a large, seven-site, five-country, population-based epidemiology study to address urgent questions about Lassa fever in advance of upcoming vaccine trials. Up to 33,000 volunteers are expected to enroll.
2021
IAVI and Scripps announce that an experimental HIV vaccine candidate (eOD-GT8 60mer) primed the immune system as the first stage in the production of bnAbs in a Phase I clinical trial (IAVI G001).
IAVI announces a joint project with EDCTP and CEPI to conduct advanced clinical development of IAVI’s Lassa fever vaccine candidate among adults and children in Liberia, Nigeria, and Sierra Leone. The international collaboration, called Lassa Fever Vaccine Efficacy and Prevention for West Africa (LEAP4WA), represents institutions in Africa, Europe, and North America.
GREAT Phase I trial (HIV-CORE 006) of a novel mosaic HIV vaccine candidate, HIVconsvX, begins.
IAVI announced the award of up to US$126 million from the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health and Human Services to develop two recombinant vesicular stomatitis virus (rVSV)-vectored filovirus vaccine candidates. This award supports preclinical activities and includes options for clinical development up to and inclusive of a Phase II clinical trial of IAVI’s rVSV Sudan ebolavirus vaccine candidate (rVSVΔG-SUDV-GP). Optional work that would continue the development of IAVI’s Marburg virus vaccine candidate (rVSVΔG-MARV-GP) that is currently supported by the Defense Threat Reduction Agency of the U.S. Department of Defense could be funded at a later date.